Native states are metastable?
06/07/11 11:48 Filed in: 2011
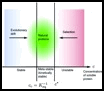
At long last, we’ve published our paper on Amyloid fibril thermodynamic stability. This paper is significant in that we have strong thermodynamic evidence that the native, functional state of a range of common human proteins is not the thermodynamically favoured state, as is conventionally thought. Instead, these proteins can lower their free energy by forming amyloid fibrils. This paper has some exciting consequences. For example, proteins must aggregate and form fibrils slowly in order for a biological organism to function. Also, kinetic mechanisms to prevent proteins from aggregating, such as molecular chaperones, are crucial for survival.
This work has been described in a commentary by Devarajan Thirumalai and Govardhan Reddy in Nature Chemistry.