A growing number of human disorders including Alzheimer’s disease (AD) and Parkinson’s disease (PD), type II diabetes and the prion diseases are associated with the deposition of proteinaceous ‘amyloid’ aggregates in human tissues. The incidence of these conditions is increasing as populations age, with an associated national cost to the UK alone larger than both cancer and heart disease combined.
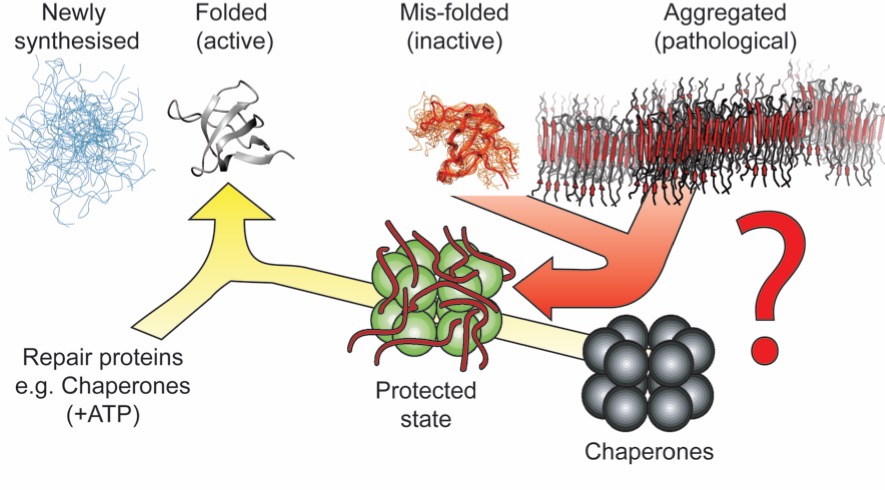
All is not lost, however! A set of proteins is up-regulated in vivo in response to the presences of such proteinacous aggregates. In vitro, these same proteins are able to dramatically reduce the tendency of proteins to aggregate. The primary focus of the lab is to better understand the mechanism by these proteins inhibit, and in some cases prevent, protein aggregation and amyloid formation.
Experimentally, this is tremendously challenging. The systems that we need to study are incredibly heterogeneous, a property that confounds almost all high resolution techniques used by modern structural biology. Our approach is to combine cutting edge NMR spectroscopy, mass spectrometry and electron microscopy measurements. By doing so, we can characterize the structures and dynamics of these heterogeneous assemblies, at atomic resolution.
The work of the group is therefore inherently inter-disciplinary. Novel experimental methodology and the computational techniques necessary to synthesize the information are developed in the group together with the molecular biology techniques required to make relevant high quality samples for detailed scrutiny. We have projects available that span theoretical, physical, biological and medicinal chemistry.
For additional details about the techniques we use, see pages on the side bar.


