The NMR experiments described above provide ‘local’ information about atomic structure and dynamics. We combine these data with measurements from ion-mobility mass spectrometry, which is able to give us three important bits of additional information: which oligomeric states are populated in solution, the timescale on which any quaternary structure rearranges and even the dimensions of individual oligomers, without the need for prior separation. By combining the local structural restraints derived from NMR, with the lower resolution but oligomer specific ‘global’ measurements, we are able to obtain a very rich picture of macromolecular behaviour.
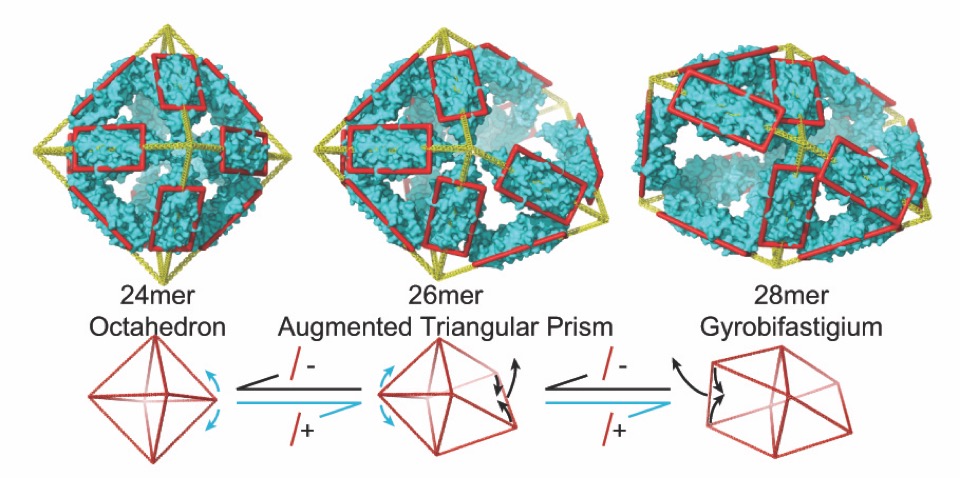
In addition to pairing our NMR measurements with ion-mobility mass spectrometer, we also employ electron microscopy. We believe the experimental triumphate of NMR, ion-mobility mass spectrometry and electron microscopy to be incredibly powerful in studies of heterogeneous mixtures, such as those that spontaneously arise in problems associated with protein mis-folding disorders. Physical chemistry to the rescue? Perhaps…


